Lung Microflora and Respiratory Tract Microflora – The Complex Interplay
Lung (pulmonary) microflora and respiratory tract microflora can directly affect immune function and diseases or can be affected by changes in immune capability and local inflammation during disease progression. Early and serve viral infections, such as infections with the respiratory syncytial virus (RSV), influenza virus, and even common cold virus, may alter the long-term immune responses of the lung, especially in infants who may not yet have a stable lung microflora.
Numerous correlational studies have suggested that changes in the lung microflora may associated with disease progression, including allergies. However, beyond cases confirmed as bacteria pneumonia, it is still uncertain whether specific members of the lung microflora directly serve as agents that trigger or modulate susceptibility to diseases or the protective capacity [1].
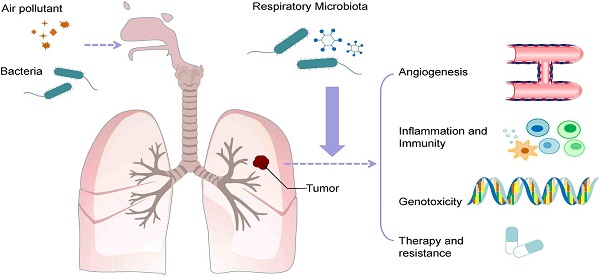
Implications for Inflammation and Immune Response
However, there have been recent reports in this field, most notably regarding the role of Prevotella spp. in the respiratory tract. Prevotella spp. is often found in the respiratory tract of healthy individuals [2-5] and exhibits differences in the lipopolysaccharide (LPS) structure on bacterial cell membrains compared to Gram-negative Gammaproteobacteria [6,7]. In a study, dendritic cells, which originated from human mononuclear cells (Hu mDCs), were cultured with Prevotella spp. or Haemophilus spp. to monitor activation signals and cytokine expression. While many bacteria could induce CD83, CD40, and CD86, Haemophilus spp.
Resulted in higher expression levels of cytokine IL-23, IL12p70, and IL-10. Moreover, the co-culture experiments with both strains have revealed that Prevotella spp. can reduce IL-12p70 induced by Haemophilus influenzae affecting Hu mDCs [7]. Similar responses also were observed in both in-vitro and in-vivo experiments [6]. In other serial studies on 112 lung-transplanted patients, it was observed that changes in the respiratory tract microflora, with Prevotella dominance, correlated with the development of inflammatory patterns or macrophage modifications [8].
The different levels of Prevotella distribution (low or high) have been documented in healthy individuals [2,5]. The higher distribution rate of Prevotella is associated with enhanced “sub-clinical” lung inflammation, notably an increased expression of pro-inflammatory cytokines and increased Th-17 lymphocyte activity [5,9]. However, combined studies have nit determined the direction of the cause-and-effect relationship, and this data would be most fitting with a model in which sub-clinical respiratory inflammation leads to a higher prevalence of Prevotella.
Lung Microflora Dynamics
The support for this model is observed in patients with fibrosis, indicating the invasion of P. aeruginosa, a type of inflammatory bacteria, significantly increases the likelihood of Prevotella appearing [10]. However, as previously described, chronic respiratory tract infection is ultimately related to the presence of the Gammaproteobacteria population, which is dominant in the respiratory tract [1,6,7]. The connection between inflammation and microflora is a two-way relationship in the context of lung inflammation in mice treated with LPS/elastase (a model of chronic obstructive pulmonary disease), as it has been reported to increase Pseudomonas spp. and decrease Prevotella spp.
The instillation of bronchoalveolar lavage fluid from diseased mice through the nasal route, containing lung microflora, helps enhance the production of IL-17A in the lungs of treated individuals, whether they receive antibiotic treatment or are germ-free [11]. This is consistent with findings that targeting the lung microflora by innate immune defenses, through a deficiency in plgR/slgA, leads to regeneration of the progressing small airways and emphysema [12]. In general, these observations support a novel model suggesting that the interaction of the lung immune system with microflora originating from the mouth (especially Prevotella spp.) during a healthy state is part of the central homeostatic process regulating pulmonary inflammation responses.
Many studies have demonstrated that the composition of gastrointestinal tract microflora can influence the development of immune responses in the lung, such as allergic respiratory disease [13-17]. For example, in the study of Trompette and colleagues, mice fed a high-fiber diet increased the levels of short-chain fatty acid (SCFAs) in circulation and were protected against allergic lung inflammation, while a low-fiber diet reduced SCFA levels and increased allergic respiratory disease [16].
Deciphering the Gut-Lung Axis: Microbial Metabolites and Immune Responses
This immune regulation mechanism is associated with changes in bone marrow hematopoiesis characterized by enhanced production of precursors of granulocytes and dendritic cells (DCs) and subsequently high Th2 cell-promoting DCs with decreased Th2 cell-driving capacity. Based on observations in mouse models over a decade ago, the underlying mechanisms of the gut-lung immune regulatory axis remain incompletely elucidated.
Although pathogen-associated molecular patterns (PAMP) from various microorganisms can contribute to the nature of disease-causing innate immune responses, these microorganisms themselves produce intermediate compounds that can alter subsequent immune responses. The impact of metabolites originating from bacteria on the nature of immune responses has been reported in several studies of the gut microflora.
For example, the relationship between diet and the microflora to regulate immune responses has been established with fiber and Clostridium sp. leading to the production of short-chain fatty acids and the development of Treg cell [18-22]. Other members of the gut or lung microflora may provide protective intermediates, including anti-inflammatory omega-3 polyunsaturated fatty acids (ω-3 PUFA) and immune-regulating tryptophan metabolites [23,24]. Therefore, understanding the resident microbial groups in the lungs during health and disease, as well as the metabolites they produce, will help to paint a clearer picture of the immune environment and regulatory responses.
References
- Dickson, R.P., Erb-Downward, J.R., Martinez, F.J. & Huffnagle, G.B. The microbiome and the respiratory tract. Annu. Rev. Physiol. 78, 481–504 (2016).
- Bassis, C.M. et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio 6, e00037 (2015).
- Morris, A. et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care. Med. 187, 1067–1075 (2013).
- Venkataraman, A. et al. Application of a neutral community model to assess structuring of the human lung microbiome. MBio 6, e02284–14 (2015).
- Segal, L.N. et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 1, 19 (2013).
- Bernasconi, E. et al. Airway microbiota determines innate cell inflammatory or tissue remodeling profiles in lung transplantation. Am. J. Respir. Crit. Care. Med. 194, 1252–1263 (2016).
- Larsen, J.M., Musavian, H.S., Butt, T.M., Ingvorsen, C., Thysen, A.H. & Brix, S. Chronic obstructive pulmonary disease and asthma-associated Proteobacteria, but not commensal Prevotella spp., promote Toll-like receptor 2-independent lung inflammation and pathology. Immunology 144, 333–342 (2015).
- Larsen, J.M. et al. Divergent pro-inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLOS ONE 7, e31976 (2012).
- Segal, L.N. et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat. Microbiol. 1, 16031 (2016).
- Tunney, M.M. et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am. J. Respir. Crit. Care. Med. 177, 995–1001 (2008).
- Yadava, K. et al. Microbiota promotes chronic pulmonary inflammation by enhancing IL-17A and autoantibodies. Am. J. Respir. Crit. Care Med. 193, 975–987 (2016).
- Richmond, B.W. et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat. Commun. 7, 11240 (2016).
- Herbst, T. et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 184, 198–205 (2011).
- Noverr, M.C., Falkowski, N.R., McDonald, R.A., McKenzie, A.N. & Huffnagle, G.B. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73, 30–38 (2005).
- Noverr, M.C., Noggle, R.M., Toews, G.B. & Huffnagle, G.B. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 72, 4996–5003 (2004).
- Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
- Fujimura, K.E. et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc. Natl Acad. Sci. USA 111, 805–810 (2014).
- Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
- Zeng, H. & Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 36, 3–12 (2015).
- Kosiewicz, M.M., Dryden, G.W., Chhabra, A. & Alard, P. Relationship between gut microbiota and development of T cell associated disease. FEBS Lett. 588, 4195–4206 (2014).
- Geuking, M.B., McCoy, K.D. & Macpherson, A.J. Metabolites from intestinal microbes shape Treg. Cell. Res. 23, 1339–1340 (2013).
- Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
- Memari, B. et al. Engagement of the aryl hydrocarbon receptor in mycobacterium tuberculosis-infected macrophages has pleiotropic effects on innate immune signaling. J. Immunol. 195, 4479–4491 (2015).
- Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).